What Is the Solute and Solvent in Bleach
The substance in which a solute dissolves to produce a homogeneous mixture. What is the solvent in a liquid liquid solution.

Chapter 8 Solutions 5 Glucose And 0 9 Saline Solutions Are Used In Iv Therapy Ppt Download
All of these examples have both a solute and a solvent.
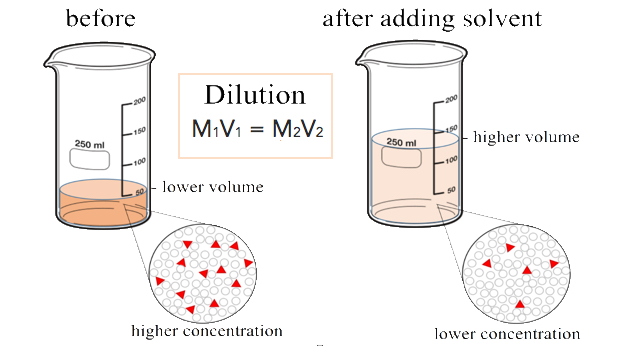
. There are numerous examples of solutions. Examples of solutes include sugar salt oxygen etc. So in the salt water example the salt is the solute and the water is the solvent.
The solute is the material that is dissolved while the solvent is whatever it is dissolved in. Is vinegar an example of solute. Solute A solute is something that is dissolved in a solvent.
The solute is the substance that is being dissolved while the solvent is the dissolving medium. The solute although there is more than one is sodium hypochlorite which is what makes it bleach. There will always be less solute than solvent.
The solute is present in a lesser amount that the solvent. In vinegar acetic acid is the solute and water is the solvent and in bleach sodium hypochlorite is the solute and water is the solvent. The substance that dissolves it is called the solvent.
When the solute dissolves it separates into individual particles which spread throughout the solvent. While all the above examples are what chemists call aqueous solutions where water is the solvent there are other types of solutions you encounter daily. A solute is a molecule or particle that is distributed in a solvent.
Similarly bleach is a solution of sodium hypochlorite. All of these examples have both a solute and a solvent. Is Vinegar a solute or solvent.
Examples of Solutes Solvent White sugar Powdered sugar Brown Sugar Table salt Deicing salt. In vinegar acetic acid is the solute and water is the solvent and in bleach sodium hypochlorite is the solute and water is the solvent. Similarly bleach is a solution of sodium hypochlorite.
Similarly bleach is a solution of sodium hypochlorite. In vinegar acetic acid is the solute and water is the solvent and in bleach sodium hypochlorite is the solute and water is the solvent. All of these examples have both a solute and a solvent.
In vinegar acetic acid is the solute and water is the solvent and in bleach sodium hypochlorite is the solute and water is the solvent. In vinegar acetic acid is the solute and water is the solvent and in bleach sodium hypochlorite is the solute and water is the solvent. Practically the solute is also usually being added to the solvent.
If a solution contains 20 g of solute dissolved in 05 L of water what is the percentage of this solution. Is bleach a solute or solvent. What is the dilution factor if.
The solute is isopropyl alcohol and the solvent is water. So in the salt water example the salt is the solute and the water is the solvent. Is bleach a solute.
What is the solvent and solute of bleach. Is bleach considered a solvent. What is the.
Is oil a solute. Is Vinegar an example of solute. In vinegar acetic acid is the solute and water is the solvent and in bleach sodium hypochlorite is the solute and water is the solvent.
Similarly bleach is a solution of sodium hypochlorite. What is a solute and solvent. Bleach is already a 10 solution in the original bottle.
That means in a solution the solute is always the minor component. The substance that dissolves is called the solute. In vinegar acetic acid is the solute and water is the solvent and in bleach sodium hypochlorite is the solute and water is the solvent.
The solvent in bleach is water. The solutes are very different and each company has many recipes. For example milk solvent and sugar solute makes sweet milk.
The substance that dissolves in a solvent to produce a homogeneous mixture. All of these examples have both a solute and a solvent. The solute is the material that is dissolved while the solvent is whatever it is dissolved in.
The part of a solution that is present in the greatest amount. In soda pop high-fructose corn syrup or other sweeteners flavorings and carbon dioxide CO2 gas are the solutes and water is the solvent. For example for Colgate Plax the ingredients are.
Ammonia in any of these cleaners would be the solvent. When you can add more solute which continues to dissolve your solution is. What is the solute and solvent of soft drink.
In vinegar acetic acid is the solute and water is the solvent and in bleach sodium hypochlorite is the solute and water is the solvent. A solute can take many forms. Household Solutes and Solvents By.
Grams of solute Volume of solvent100. Is bleach a solute. It may be in the form of a gas a liquid or a solid.
The solute is the material that is dissolved while the solvent is whatever it is dissolved in. A solute is a substance that can be dissolved into a solution by a solvent. In vinegar acetic acid is the solute and water is the solvent and in bleach sodium hypochlorite is the solute and water is the solvent.
In vinegar acetic acid is the solute and water is the solvent and in bleach sodium hypochlorite is the solute and water is the solvent. Unlike bleach acetone is a solvent and is miscible. In vinegar acetic acid is the solute and water is the solvent and in bleach sodium hypochlorite is the solute and water is the solvent.
Conner McGill Solvent A solvent is a substance that is able to dissolve other substances. So in the salt water example the salt is the solute and the water is the solvent. Volume of solventGrams of solute 100 d.
The oil carries some solute which can. Bleach which is used to disinfect laundry is the solid compound sodium hypochlorite the solute dissolved in water the solvent. What happens if you add more solute to a supersaturated solution.
In vinegar acetic acid is the solute and water is the solvent and in bleach sodium hypochlorite is the solute and water is the solvent. Ammonia N H 3 N H 3 is a strong cleaning agent a base and is often the main ingredient of window cleaners and disinfectants. In vinegar acetic acid is the solute and water is the solvent and in bleach sodium hypochlorite is the solute and water is the solvent.
Is oil a solute or solvent. Grams of solute Volume of solvent 100 c. The solvent is water or ethanol or their mixture.
While all the above examples are what chemists call aqueous solutions where water is the solvent there are other types of solutions you encounter daily. Typically the solute will be uniformly distributed in the solvent after mixing. The solute is present in.

What Is The Molarity Of A Bleach Containing 9 5 Grams Of Naocl Per Liter Of Bleach How Many Grams Of Cacl 2 Would Be Dissolved In 0 75 L Of Water Ppt Download

Answered 1 Bleach Solution Contains The Solute Bartleby

Bleach Water Plastic Bottle Concentrate Solvent In Chemical Reactions Cleaning Perfume Cleaning Png Pngegg

Preparing And Diluting Solutions Ppt Download
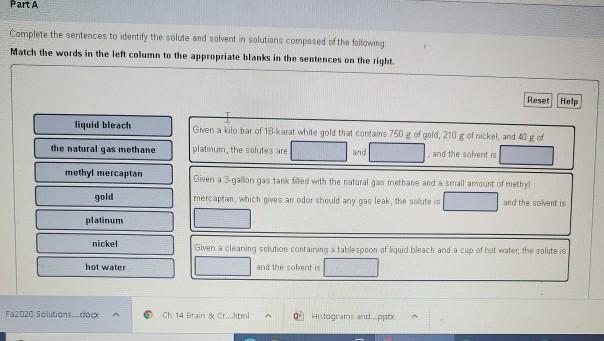
Solved Part A Complete The Sentences To Identify The Solute Chegg Com

9 3 Concentration Chemistry Libretexts
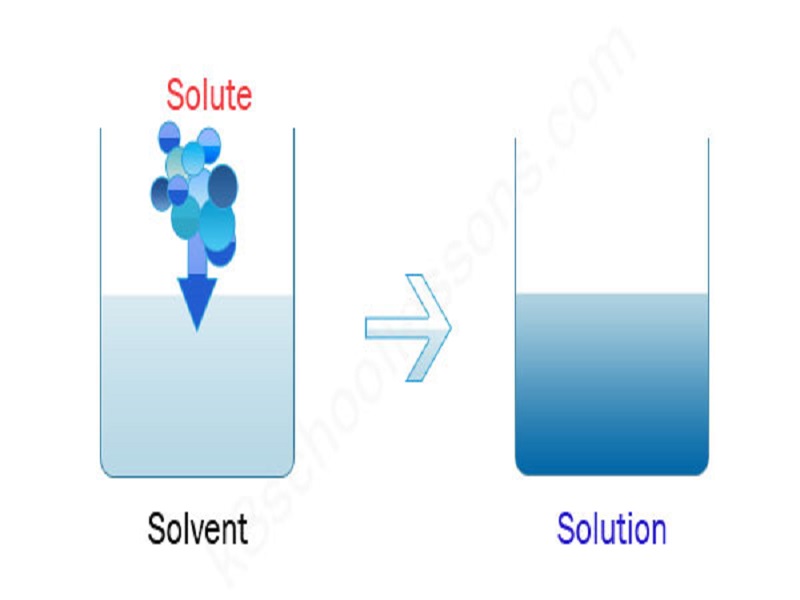
8 Solute Examples In Everyday Life Studiousguy
Solvent Science About Cleaning Products

Other Units For Solution Concentrations Chemistry Atoms First
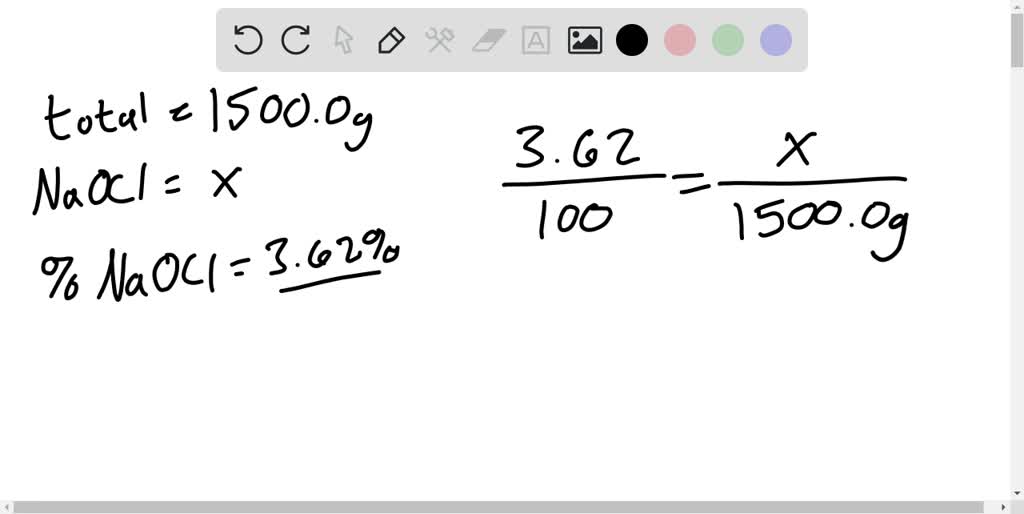
Solved You Have 1500 0 G Of A Bleach Solution The Percent By Mass Of The Solute Sodium Hypochlorite Naocl Is 3 62 How Many Grams Of Naocl Are In The Solution
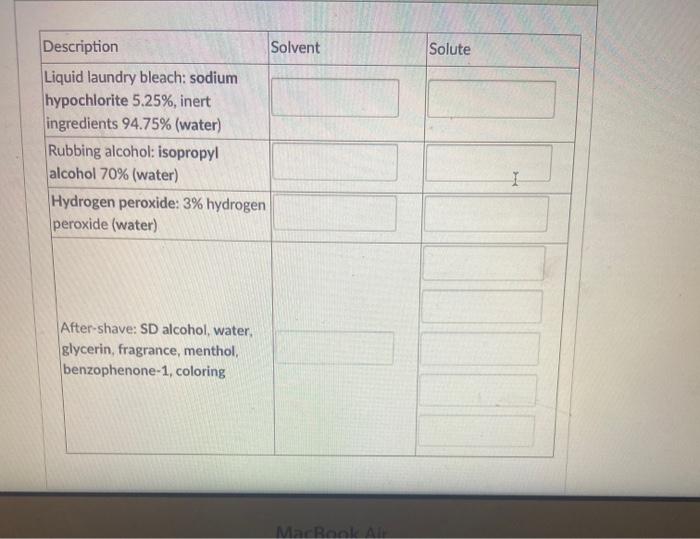
Solved Solute Description Solvent Liquid Laundry Bleach Chegg Com

What Is A Solvent Definition Examples Video Tutors Com

Concentrations Of Solutions Ppt Download

Solvent Solute Solutions And Solubility Matter Pure Substances Elementscompoundsmixturessolutionssuspensions Ppt Download

What Is The Role Of A Solvent In A Chemical Reaction




Comments
Post a Comment